About
The Epigenomics and Mechanisms Branch (EGM) conducts hypothesis-driven studies as well as data-driven mechanistic studies with a special emphasis, aimed at advancing the understanding of cancer causation and the mechanisms of tumorigenesis and promoting international collaboration, the core activities of IARC. EGM’s ambition is also to place more emphasis on contributions to translational studies, through the discovery of mechanism-based biomarkers of exposure and risk stratification. EGM’s studies are interdisciplinary in nature, and the major EGM programmes promote and advance synergistic collaborations with other IARC laboratory-based scientists, epidemiologists, and evidence synthesis experts, as well as with numerous international collaboration groups.
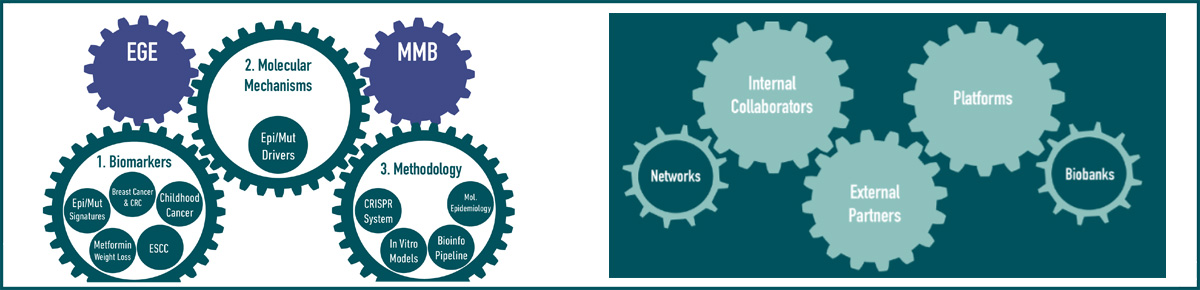
As outlined in Figure 1, EGM combines molecular epidemiology and experimental studies to investigate the roles of (epi)genomic and other molecular changes and deregulated pathways induced by environmental or lifestyle factors in cancer development, and to identify cancer biomarkers. EGM builds on existing and new collaborative work on human biospecimens using genomic platforms, molecular and cell biology, and biochemistry tools. EGM also develops relevant innovative methodologies, molecular profiling strategies, cancer modelling systems, and bioinformatics tools, applicable to population-based cohorts and molecular epidemiology studies coordinated by IARC researchers and external collaborators.
Strategic vision
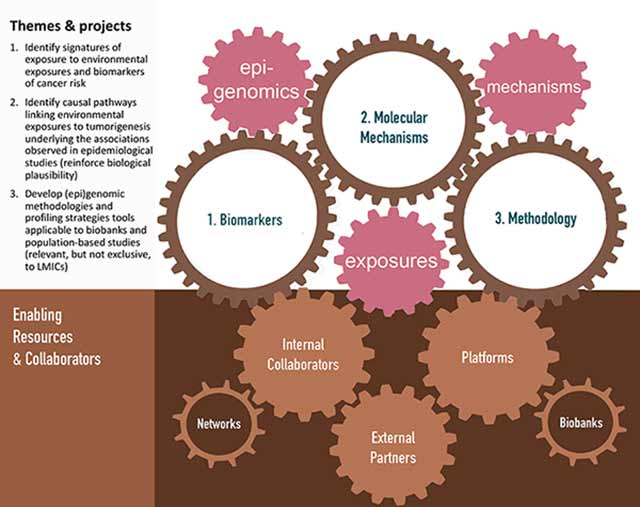
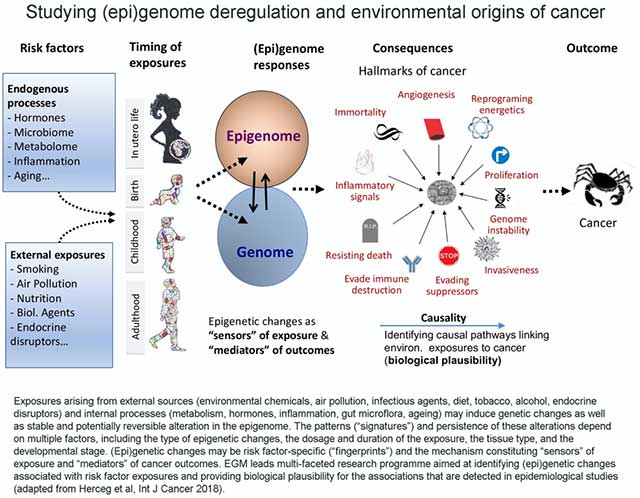
Identifying the causal pathways that link environmental or lifestyle exposures to tumorigenesis and gaining insights into the molecular mechanisms that underlie the associations observed in epidemiological studies (biological plausibility) provide a foundation for studies of cancer etiology, carcinogen evaluation and classification, and ensuring evidence-based cancer prevention (Figure 2).
The overarching objective of EGM is to advance the understanding of cancer causes and of the underlying mechanisms of carcinogenesis, and thus enrich the evidence base for cancer prevention. This is achieved by exploiting conceptual and technological advances in molecular and cell biology. EGM’s ambition is to remain at the forefront of the field of laboratory science and molecular epidemiology by adopting new conceptual and technological advances and by capitalizing on IARC’s unique role in international cancer research. This includes, for example, CRISPR/Cas9 and dCas9-manipulated two-dimensional and three-dimensional cell exposure models such as organoids, somatic genetics (with a focus on mutational signatures and DNA damage signalling as opposed to genome-wide association studies or analysis of single-nucleotide polymorphisms), and epigenetics (with a focus on DNA methylation and modulations of chromatin and of the transcriptome).
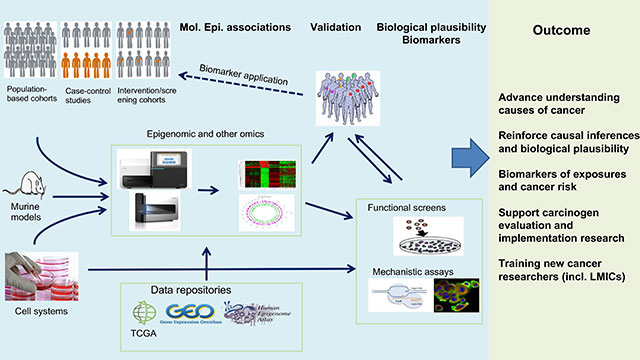
Key elements of EGM’s strategy include developing innovative state-of-the-art molecular and cell biology and functional epigenomics research methodologies and bioinformatics and biostatistics tools, applicable to experimental cancer models and human samples from biobanks associated with population-based and case–control studies (Figure 3). EGM’s ambition is also to place more emphasis on contributions to translational studies, through the discovery of mechanism-based biomarkers of exposure and risk stratification.
Role of EGM within IARC
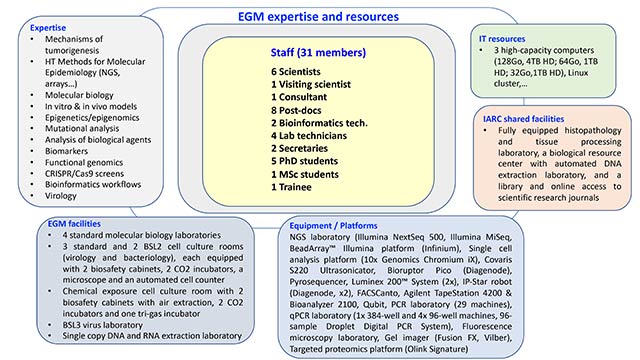
EGM’s unique position within IARC draws on its laboratory-based and data science expertise and cutting-edge experimental research tools (Figure 4), which make an essential contribution to IARC’s mission, aimed at advancing the understanding of causes of cancer and the application of biomarkers to implementation research and prevention. Mechanistic evidence and reinforcing biological plausibility are instrumental in addressing new etiological hypotheses, and EGM scientists are uniquely positioned for this.
EGM’s research is focused on areas where it can fill important knowledge gaps in cancer etiology and inform cancer prevention, while complementing and reinforcing other major research programmes carried out by IARC. Therefore, the research in EGM provides opportunities to investigate emerging cancer risk factors and their underlying mechanisms, some of which may leave specific epigenetic or mutational signatures that provide valuable molecular markers and opportunities for improving cancer prevention.
EGM also develops powerful cutting-edge tools and methodologies that are directly applicable in epidemiological studies, offering improved measurement of exposure, and risk stratification, all of which are priority areas for IARC. Thus, by providing unique expertise, skills, and research tools, EGM enables IARC to rely on integrative approaches that incorporate molecular mechanisms, functional (epi)genomics, bioinformatics, and fieldwork epidemiology, as well as biobanks coordinated by IARC epidemiologists.
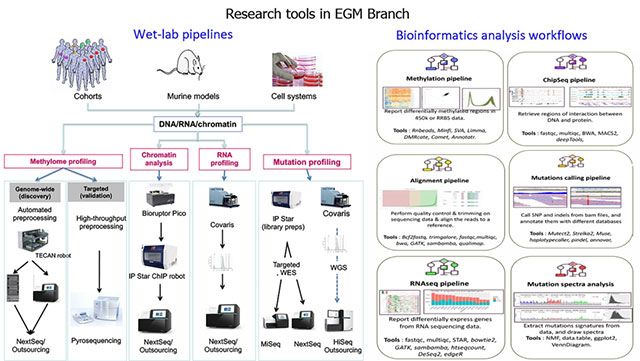
Given that the demand for affordable and less labour-intensive strategies is increasingly met by emerging technologies that enable robust and cost-effective (epi)genome analysis, the adoption of suitable methodologies and analytical tools applicable to human samples as well as experimental models is an essential part of EGM’s strategy (Figure 5). EGM is involved in the development of translational studies (such as those involving interventions aimed at reducing cancer risk or recurrence) and in cancer research that is relevant to, although not exclusive to, low- and middle-income countries. This strategic vision supports the transition between the IARC Medium-Term Strategy for 2016–2020 and the Medium-Term Strategy for 2021–2025.
Considering these objectives and opportunities, EGM contributes to IARC’s overall mission by extending the current knowledge of cancer etiology and by providing the evidence base for carcinogen evaluation and cancer prevention. EGM’s strategy builds on its strengths and recent achievements and capitalizes on opportunities brought about by conceptual and technological advances in the fields of laboratory science, molecular epidemiology, and computational science. For current and future work, EGM is focusing on three priority areas: (i) environmental exposures, (epi)genetic changes, and cancer risk throughout the life-course, (ii) the functional role of genetic and epigenetic changes in carcinogenesis, and (iii) obesity, epigenetic changes, and cancer.
EGM gratefully acknowledges funding from the European Union, the National Institutes of Health–National Cancer Institute (USA), the Institut National du Cancer (INCa, France), and international and national cancer charities: Association pour la Recherche sur le Cancer (ARC, France), Canadian Institutes of Health Research (CIHR, Canada), Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail (ANSES, France), Institut national de la santé et de la recherche médicale (INSERM, France), Worldwide Cancer Research (United Kingdom), New Frontiers in Research Fund (Canada), World Cancer Research Fund International, Research Foundation Flanders (FWO, Belgium), Cancer Research UK, and Ligue nationale contre le Cancer (France).